The answer is, surprisingly, maybe.
You might be familiar with the use of red or purple coloured leaves or flowers as a test for pH – I used red cabbage leaves in the soil medium pH experiments for the post Water hardness, pH and bonsai. The pigments in these leaves and flowers (usually anthocyanins – read more about them here) respond to pH by changing colour quite predictably. But how leaves or petals behave in a dish with acid or alkali bears no relation to how they develop as organs of the plant.
I came into this post with the hypothesis that pH would not affect flower colour. The internal environment of all cells – including those of plants – is actively regulated and buffered to avoid variation – a process known as homeostasis. Cells need to maintain a stable internal environment for the biochemical reactions of life to operate effectively. Key cellular attributes like temperature, pH, salinity, glucose levels, metals and other nutrients are carefully balanced, and cells have specific mechanisms to adjust these up and down within a band of tolerance. So adding acid or alkili to a plant’s growing medium doesn’t make their cells more or less acidic, and if that doesn’t happen then it wasn’t clear to me how the pigments in the cell vacuoles could react with anything to change colour.
But it turns out that what can end up changing a plant’s flower colour is the presence of metal ions. If you’ve read my post on nutrients, or probably even if you haven’t, you’ll know that a certain amount of trace metals are necessary for biologically essential processes, many of which include metal ions in the relevant enzymes (like RuBisCO which has a Magnesium ion and is a crucial enzyme in photosynthesis). Plants need these metals, but overdoses of essential elements negatively affect plant metabolism, so they have evolved mechanisms to handle excess amounts.
One of these mechanisms is chelation. Chelation is when a metal ion reacts with another molecule to form a complex, taking the metal out of its reactive, ionic state. Metal ions chelate with pigment molecules in plants such as anthocyanins and flavonoids, creating compounds known as metalloanthocyanins, or metal-flavonoids. The formation of these compounds shifts the colour spectrum previously displayed by the pigment.
A variety of metal ions have been shown to form these complexes, including aluminium (Al3+), iron (Fe3+), magnesium (Mg2+), and calcium (Ca2+). Laboratory studies have also shown that other ions, such as cobalt (Co3+), manganese (Mn2+), zinc (Zn2+), and cadmium (Cd2+), can produce various colours when chelated with anthocyanins.
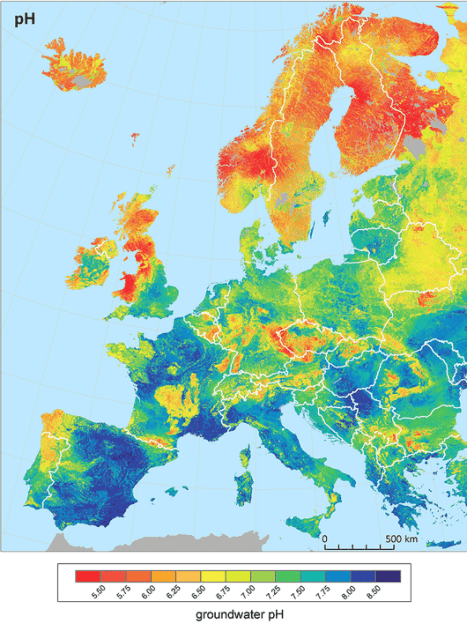
The key relationship with pH is that these positively charged metal ions are more bioavailable to plants in acidic soils. This means in acid soil more positively charged metal ions (if present) are taken up into the plant, when excess amounts are dealt with through chelation with pigment molecules.
Some examples are (left to right below), Hydrangea (goes blue with Aluminium in an acidic environment), Camellia japonica (some cultivars produce purple flowers through Aluminium chelation), Camellia chrysantha (deep yellow colour comes from Aluminium chelation with quercetin flavonoids), and Centaurea cyanus (Cornflower) which requires Iron and Magnesium for its blue colour, as do Petunias.




The addition of magnesium has been shown to increase the content of anthocyanins and enrich the flower colours of various ornamental plants including Anigozanthos flavidus (red flowers), Limonium sinuatum (blue bracts), Gypsophila elegans (pink flowers) and Aconitum carmichaelii (blue flowers). Magnesium supplementation in grapevines has also been observed to brighten grape colour.
Obviously not many of these are common bonsai species. In theory, based on the information in this post, trees with pink flowers such as hawthorn, azalea, cherry and even the leaves of Japanese Maples could operate on similar principles. Azaleas and Japanese Maples are thought to be less vigorous and colourful in alkaline soils, but I haven’t found any evidence linking this specifically to metal chelation. So for sure if you have a Hydrangea bonsai then this will change colours based on pH – and the presence of Aluminium. But for the rest there isn’t any evidence for or against. If anyone would like to do some experiments and share their results, please get in touch.
Editor’s note: for the first time I used Google Deep Research to assist with writing this article. As a result the references don’t line up to exact sentences like in my other posts. I’m not sure I like this, and may change my approach for future posts, but in the meantime below are the references which were relevant to the content I ended up using.
- Function of Anthocyanin and Chlorophyll Metabolic Pathways in the Floral Sepals Color Formation in Different Hydrangea Cultivars
- (PDF) Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition
- Anthocyanin stability and degradation in plants – PMC
- Curious Chemistry Guides Hydrangea Colors | American Scientist
- A Genomic Approach to Study Anthocyanin Synthesis and Flower Pigmentation in Passionflowers – PMC
- Acid or base? How do plants regulate the ecology of their phylloplane? | AoB PLANTS | Oxford Academic
- Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses – PMC
- The Mechanism of Metal Homeostasis in Plants: A New View on the Synergistic Regulation Pathway of Membrane Proteins, Lipids and Metal Ions
- Characteristics of Aluminum Accumulation and Distribution in Leaves of Different Ages in Camellia oleifera
- Aluminum Ions Are Involved in Purple Flower Coloration in Camellia japonica ‘Sennen-fujimurasaki’
- Molecular Basis of Aluminium Toxicity in Plants: A Review
- Extraction of Anthocyanins from Black Grape By-Products and Improving Their Stability Using Cobalt(II) Complexation – PMC
- Increased anthocyanin accumulation in ornamental plants due to magnesium treatment | Request PDF
- Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions – PMC